abbott point of care covid test
Panbio COVID-19 Antigen Self-Test packaging is up to 33 smaller and 20 lighter than the original packaging. The ID NOW COVID-19 test returns positive results in 13 minutes or less to enable immediate clinical decisions during the first patient visit.

Abbott Panbio Covid 19 Antigen Rapid Test Diepe Neustest 25x Hulptroepen
Ad Save 20-40 on medical supplies with bttn when you automate your medical ordering today.
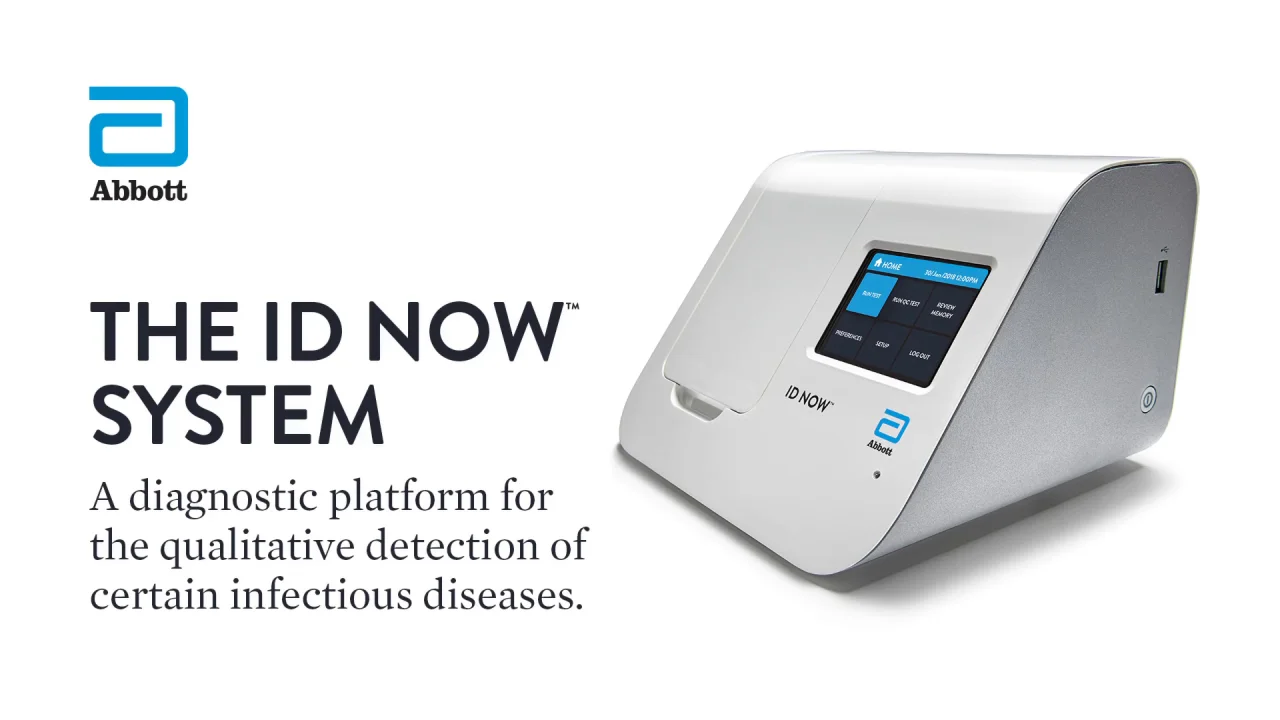
. The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less. June 17 2022 joliet police department officers california pinot noir ratings. It is used on our ID NOW platform.
Panbio COVID-19 A g Rapid Test Device. The COVID-19 test which is the fastest available molecular point-of. Abbott to market starting next week a fast point-of-care coronavirus test delivering positive results in 5min and negative results.
Our rapid antigen test BinaxNOW COVID-19 Ag Card Home Test and Self Test all provide results in 15 minutes. According to Abbott the rapid test which runs on the ID NOW platform. Abbott covid test false positive rate.
Abbott to market starting next week a fast point-of-care coronavirus test delivering positive results in 5min and negative results in 13min. CLIA-certified laboratories or testing sites are no longer required to report negative results for non-NAAT. A box containing a 5-minute test for COVID-19 from Abbott Laboratories is pictured during the daily briefing on the novel coronavirus in the.
The Abbott ID NOW COVID-19 test brings rapid testing to a wide range of front-line healthcare environments such as physicians offices urgent care clinics and hospital emergency departments. Food and Drug Administration FDA for the ID NOW COVID-19 test in March 2020. For more information on ID NOW check out this article.
Abbott has rapid point-of-care solutions to support your COVID-19 and influenza testing needs. ABBOTT ID Now COVID-19 POINT OF CARE TESTING 5192020 The following guidance is for institutions that have an Abbott ID NOW instrument and test kits for performing CLIA-waived rapid point of care COVID-19 testing. Abbott Laboratories ABT announced the receipt of the FDAs Emergency Use Authorization EUA for its molecular point-of-care test ID NOW COVID-19 for the detection of the novel coronavirus.
This removes an entire step of the testing process making it more efficient and easier to follow. Abbotts new point-of-care test for the novel coronavirus that causes COVID-19 was approved by the US. Discover the future of medical supply bttn is democratizing supply prices for businesses.
We shortened the nasal swab minimizing waste while also decreasing. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that delivers results within minutes allowing healthcare professionals to make clinical decisions during a patient visit. Abbott received emergency use authorization EUA from the US.
For professional use only. Products Solutions. The ID NOW COVID-19 rapid test delivers high-quality molecular positive results in as little as 5 minutes targeting the coronavirus COVID-19 RdRp Gene.
High performance rapid test enables immediate treatment or isolation measures to minimize transmission. The company says it will ramp up its. The arrival of the Abbott ID NOW COVID-19 test comes a week after the company launched its Abbott m2000 RealTime SARS-CoV-2 EUA test which runs on the m2000 RealTime System located in.
Abbott Laboratories ID NOW COVID-19 point-of-care test will be shipped to hospitals care clinics and doctors offices across the country starting Wednesday. The tests are intended to identify the virus by recognizing a unique section of the coronavirus genome and amplifying that portion until theres. The ID NOW COVID-19 assay is now available for use on the ID NOW platform under US.
Our rapid molecular point-of-care test detects COVID-19 in 13 minutes or less. The companys share price increased 64 to close at 7934 on Mar 30 since the news came on Mar 27. Food and Drug Administration FDA under Emergency Use Authorization EUA.
Global Point of Care Mobile Navigation button. Allocation and distribution of instruments and test kits will be determined by Central. The company says it will ramp up its.
Abbott Laboratories ID NOW COVID-19 point-of-care test will be shipped to hospitals care clinics and doctors offices across the country starting Wednesday. We also replaced the buffer bottle with a pre-filled extraction tube. This test is used on our ID NOW instrument.
ID NOW Influenza A B 2 delivers molecular flu results in less than 13 minutes on the user-friendly. Abbotts molecular point-of-care test for COVID-19 delivers positive results in as little as five minues and negative results in 13 minutes. Accessible easy-to-deploy large-scale testing helps contain the virus spread.
A CLIA-certified laboratory or testing site must report all positive SARS-CoV-2 diagnostic and screening test results to the person who was tested or that persons healthcare provider. This joins Abbotts RealTime SARS-CoV-2 test which was approved under a EUA earlier this month as well as a growing list of companies whose diagnostic tests are being. Reporting Requirements for Rapid Testing in Point-of-Care Settings.
ID NOW is an FDA approved CLIA-waived instrument which. Will deliver 50K testsday to start. Food and Drug Administration Emergency Use Authorization EUA.
/cdn.vox-cdn.com/uploads/chorus_asset/file/19856029/IDNOW_INACTION3_macro_300dpi_1200x628.jpg)
A New Covid 19 Test Can Return Results In 5 Minutes The Verge

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News

Abbott On Twitter We Re Launching A Molecular Point Of Care Test That Delivers Positive Covid 19 Results In As Little As 5 Minutes And Negative Results In 13 Minutes Providing Information Where It Is Needed

Steps To Use Id Now Effectively Abbott Newsroom
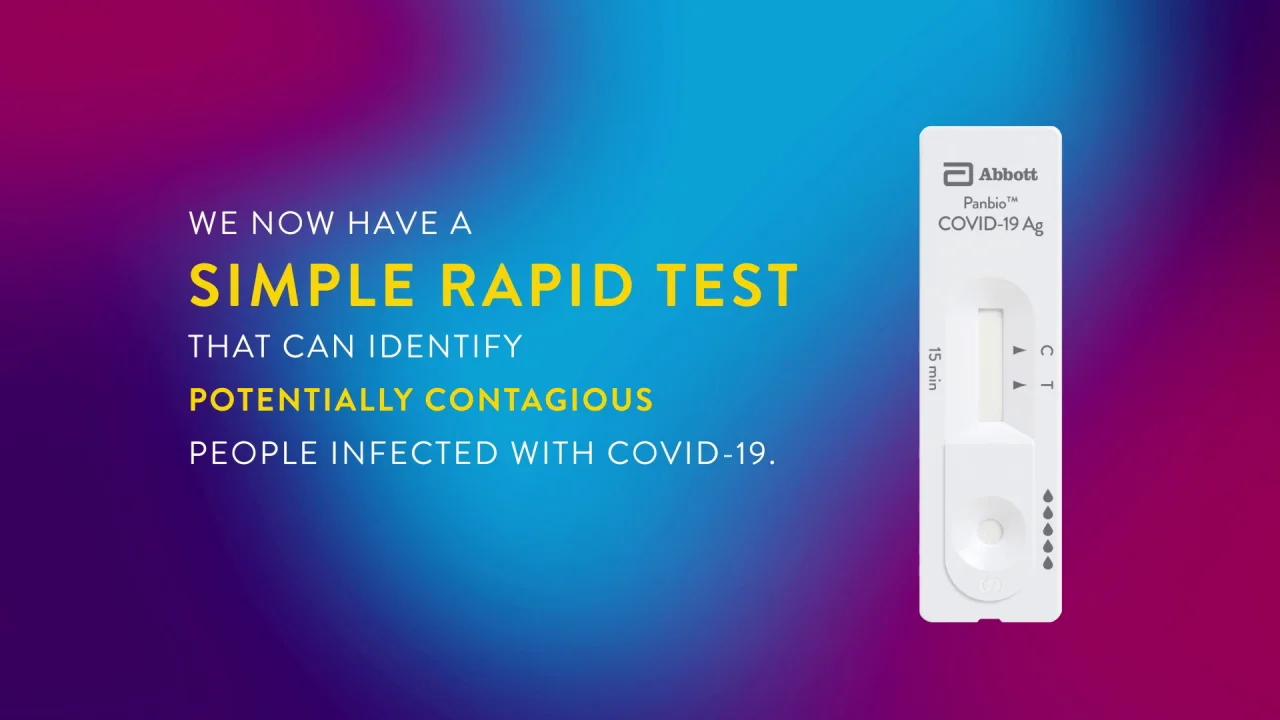
Panbio Covid 19 Ag Rapid Test Device Point Of Care Diagnose Abbott

Id Now Covid 19 Point Of Care Diagnose Abbott

Id Now Covid 19 Point Of Care Diagnose Abbott
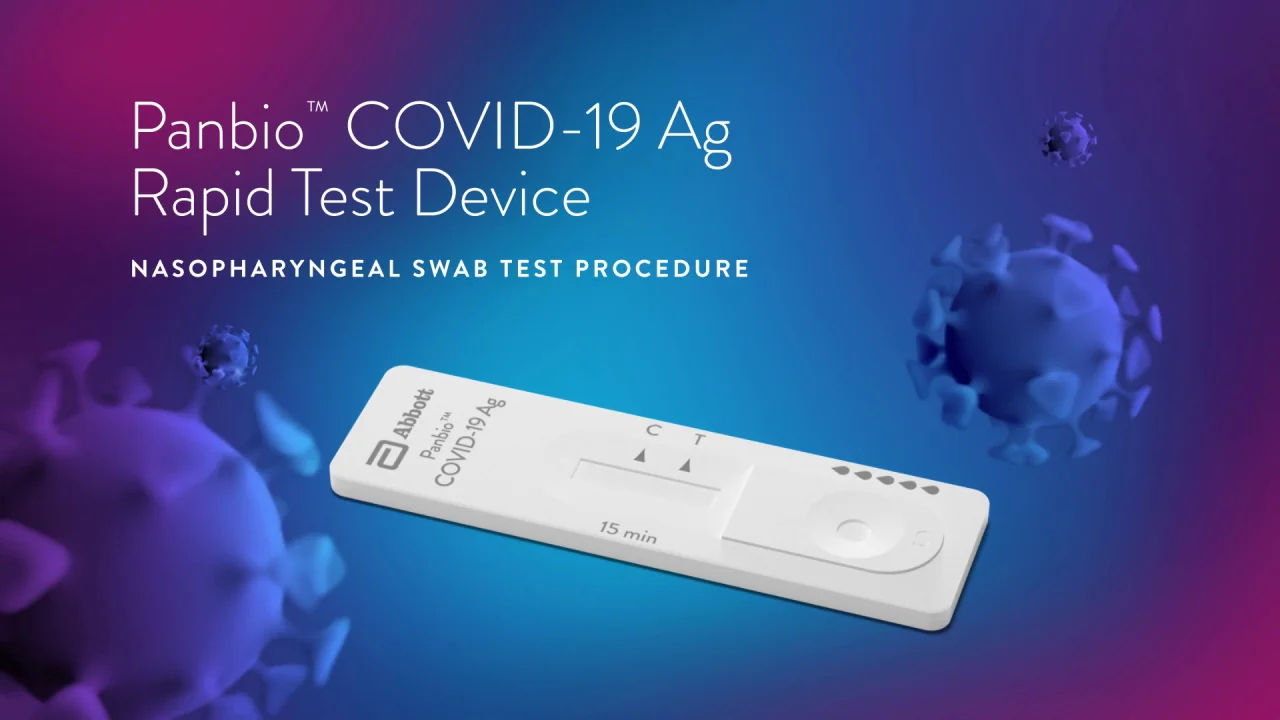
Panbio Covid 19 Ag Rapid Test Device Point Of Care Diagnose Abbott

Abbott Id Now Covid 19 Detection Test System Us
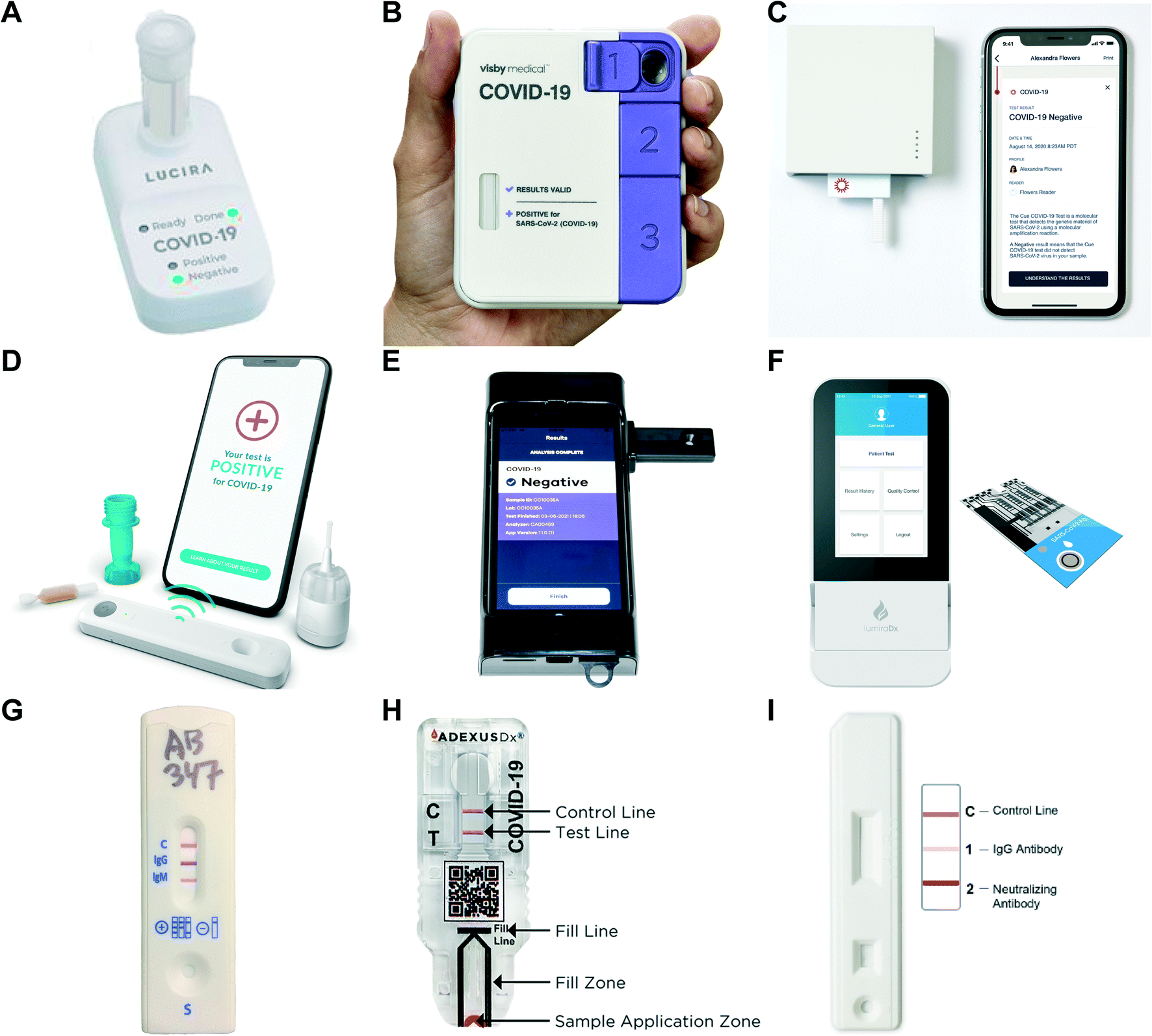
Point Of Care Diagnostics Recent Developments In A Pandemic Age Lab On A Chip Rsc Publishing Doi 10 1039 D1lc00627d

Abbott Id Now Covid 19 Instructions Modified
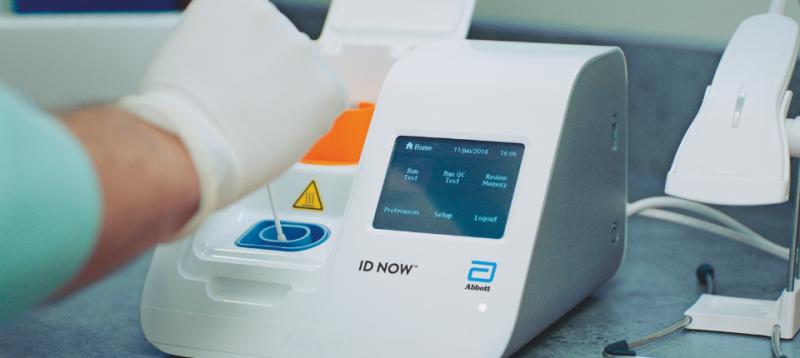
Image Gallery Showing Impact Of The Covid 19 Pandemic Daic

Point Of Care Testing Diagnostics Testing Newsroom
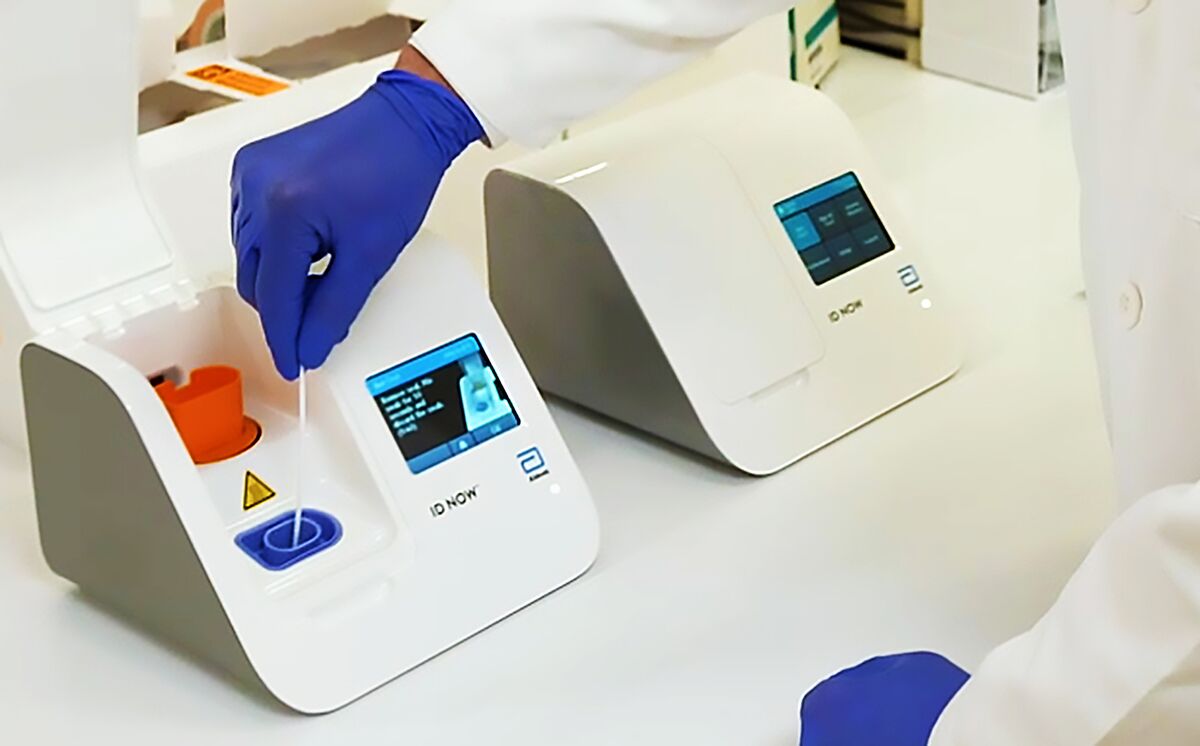
Virus News Abbott Launches 5 Minute Covid 19 Test Bloomberg

Panbio Covid 19 Ag Rapid Test Device Point Of Care Diagnose Abbott
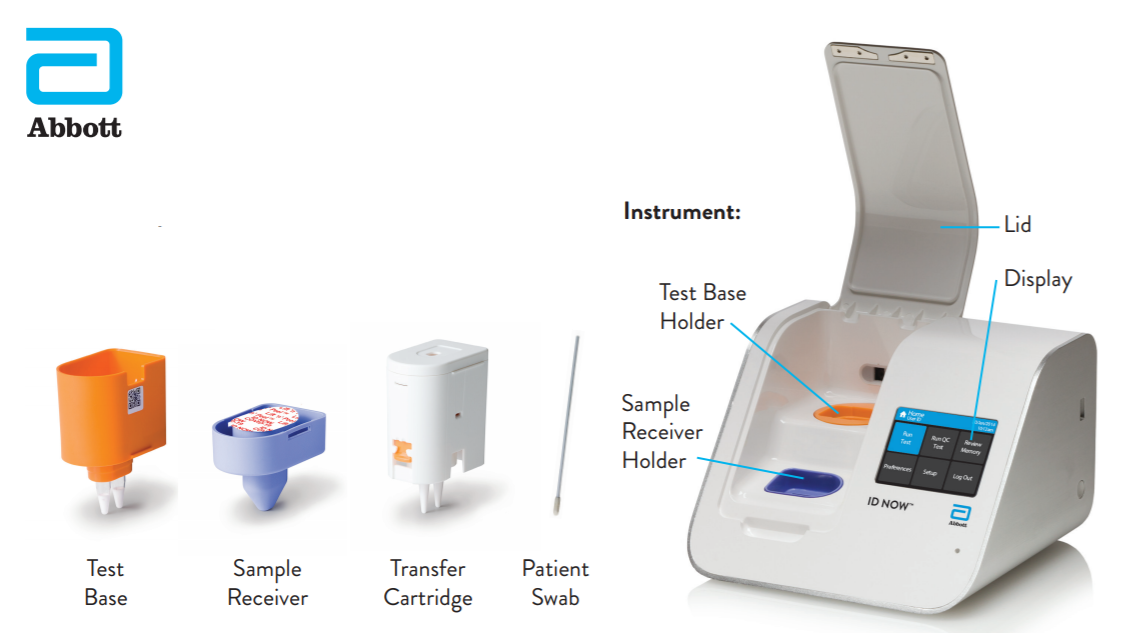
Instant Results From Abbotts Covid 19

Fda Warns About Possible Accuracy Concerns With Abbott Coronavirus Test Medical Product Outsourcing

Abbott Labs Rapid 5 Covid 19 Test To Fill In Testing Gaps For Millions In The U S
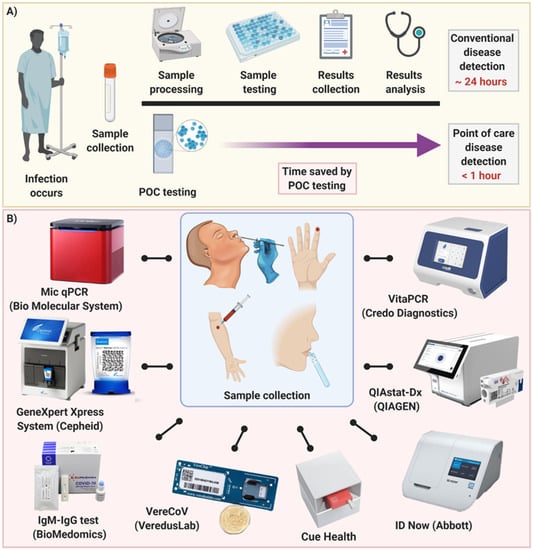
Diagnostics Free Full Text Point Of Care Diagnostics In The Age Of Covid 19 Html